Researchers from the Oxford University have began the phase 3 clinical trial of their COVID 19 vaccine in Brazil, the University announced yesterday(29th June 2020). On June 2nd the Brazilian Health Regulatory Agency (ANVISA) granted their permission to carry out Vaccine trials in Brazil, which is one of the hardest hit countries by the ongoing COVID 19 pandemic.
Since COVID 19 was first discovered in the city of Wuhan in China scientists around the world began the process of developing a vaccine against the virus. From those efforts one of the projects that seems most promising is the vaccine developed by Oxford University in partnership with AstraZeneca.
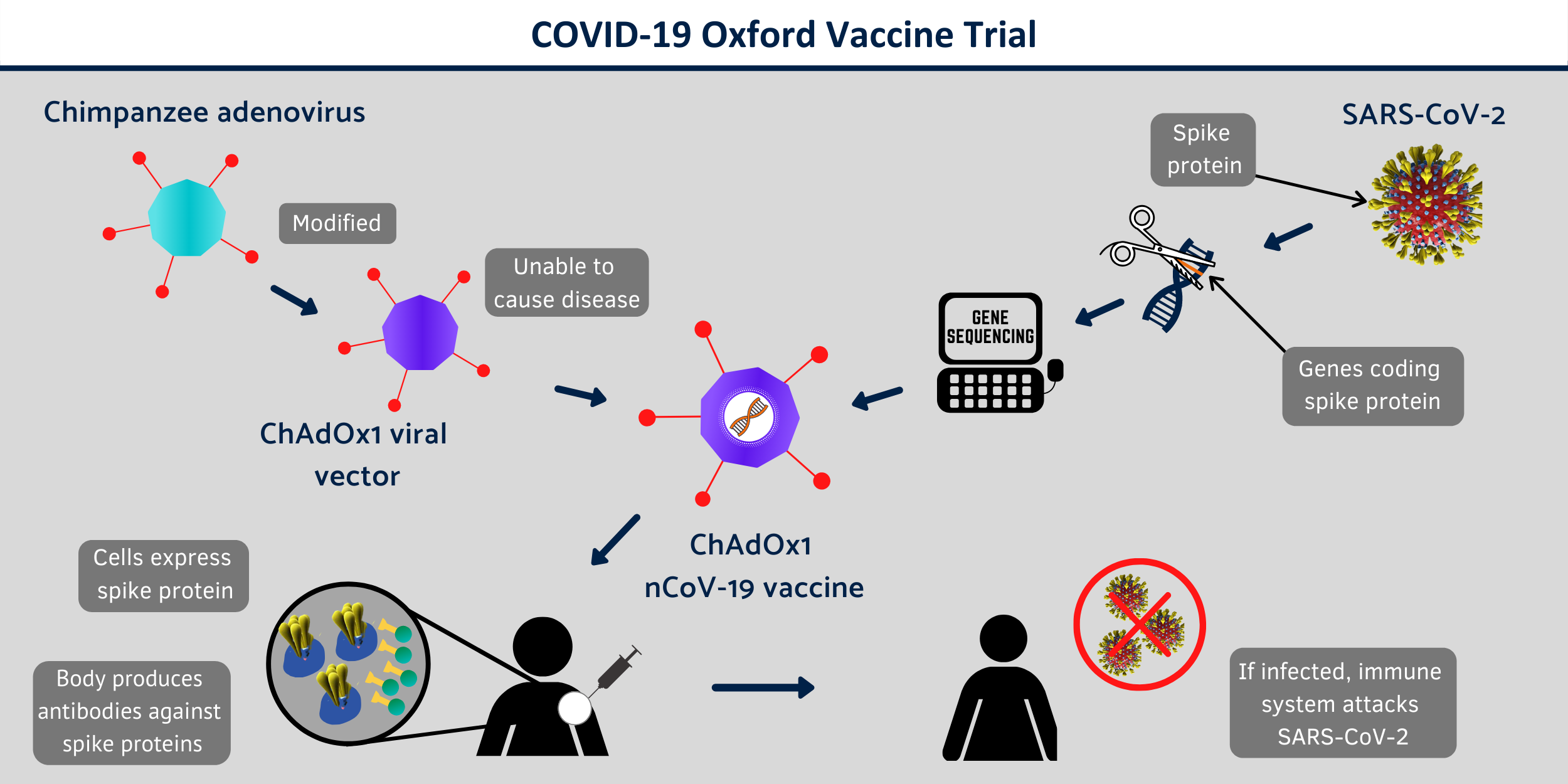
After successful completion of phase 1 and 2 the vaccine has entered phase 3 in which the vaccine is tested in actual ongoing pandemic situation using susceptible people. The technical name of the vaccine is ChAdOx1 nCoV-19, as it is made from a virus called ChAdOx1, which is a weakened and non-replicating version of a common cold virus (adenovirus). The vaccine has been engineered to express the SARS-CoV-2 spike protein.
Professor Sue Ann Costa Clemens, investigator and study coordinator from UNIFESP in Brazil, said: ‘It is an honour for me, as investigator and for my country to be selected to support the clinical development of this candidate vaccine and help the world to face a global challenge. This study has contributed to a major achievement in public health already, as the Brazilian Ministry of Health signed on June 27, an agreement for local production of ChAdOx1 nCoV-19 with AstraZeneca Brazil.’
Professor Andrew Pollard, Chief investigator of the Oxford Vaccine Trial at Oxford University said: ‘It is a privilege to be working with the researchers at the Federal University of São Paulo – UNIFESP on the first COVID-19 vaccine trial in Latin America in the next stage of this important trial.
‘The global coronavirus pandemic still presents an unprecedented threat to human health worldwide, but equally unprecedented is the impressive way researchers and scientists around the world have been able to collaborate on the clinical development work to combat this threat.’
This vaccine will help the human body to identify and make antibodies against the spike protein of SARS CoV 2 virus, which will give the human body to kill the virus during an infection. The entire world is looking at these trials as a vaccine is the only answer to this deadly virus.


