In a recent webinar organized by the Institute of Biology Sri Lanka, Prof. Neelika Malawige from the Center for Dengue Research of University of Sri Jayawardenapura delivered a special presentation covering various aspects of the COVID 19 and the SARS-CoV2 virus that are important for Sri Lanka. This article is a full transcript of her presentation.
When SARS nCoV 2 first emerged in China we knew It’s something terrible going on in China but never at that moment we thought that we too would be facing this situation today. Things became very scary when it began spreading all around the world and in some countries, there was no room in crematoriums to actually cremate the bodies. It was this scary, but still we thought, okay, this won’t happen to us and we continued our day to day lives as nothing happened. Unfortunately, today we are out there and facing the same terrible situation here in Sri Lanka. Hopefully we will never ever experience what other countries experienced over the last few months like Mass Graves with so many people dying. I strongly believe we’ll be able to control it. But just to let you know that viruses do just pop out of nowhere and cause all these pandemics. So, I just want to highlight the transmission patterns because I think it’s important to learn this. As we know that this is not the first and this is not the last that we will face another pandemic in our lifetime.
SARS-Cov2; the Virus
Coronavirus haven’t had much attention until the year 2003 because it was something that cause common cold and common cold is something that all of us have got. The first Coronavirus to grab the worldwide attention was the SARS virus in 2003, which is a recombined version of a bat virus. Later, in 2013 MERS virus appeared which was again a bat virus that transmitted to human through camels. MERS is still around but never reached Sri Lanka.
Now this particular virus named as SARS-CoV2is also believed to be originated in the bat and scientists think the intermediate host is a Pangolin. Recently there was a news from Denmark where a mutated version of SARS-Cov2 found in minks. It’s a different viral strain of this SARS Cov2 and had the ability to transmit to humans.

This SARS-CoV2 virus is looks like a crown and that’s why it’s called a coronavirus. This is an enveloped virus and because of that it is susceptible to detergents. So, all these hand washing with soap and alcohols can kill the virus easily because it dissolves the lipid envelope.
This virus is equipped with a special spike protein(S), which binds to the ACE2 receptor in human cells and initiates infection. Apart from this Spike protein there are 3 other structural protiens namely membrane protein(M), nucleic acid protein(N), Envelop Protein (E) and 16 non-structural proteins. And also, there’s a single genomic RNA molecule inside the virus with all the genomic information for the virus.
Current Situation in Sri Lanka
After the detection of the first individual with SARS CoV-2 infection within the island we saw about 60 cases in the so called first wave. Then the country went to a very prolonged two-month lockdown and the situation became under control even with few clusters like CMC cluster, Navy Cluster, Middle East returnees cluster and Kandakadu Cluster.

The current outbreak emerged at the beginning of October. And the number of infections resulted in this outbreak have gone past 14000 with over 40 deaths reported so far.
So many Questions!
With this current outbreak happening in Sri Lanka, many questions and uncertainties began to arise within the society. Those include,
- When and how is the virus transmitted?
- When are you likely to get infected if you’re exposed to somebody?
- How long would you transmit the virus to your loved ones if you are a person infected with covid?
With these and many more questions, a lot of research has been started on in this area because we’ve sort of been dealing with this virus for 10 months now.
The complete virus genome sequence was first released to the World on the 21st of January and since then many scientists from so many countries began actively working on this.
Antibody and the integer response
After the virus enters the body there’s a variable incubation period, which varies from around three to four days to 14 days. Most people show symptoms or shed the virus three to four days after infection.
One of the most critical things about this virus is that people start shedding the virus about 48 hours before they show symptoms. So, a person is infectious 24-48 hours before they show symptoms and they continue to be infectious for are about 7 to 8 days and sometimes 10 days. But as the time goes by from the onset of symptoms, the amount of infectious viral particles in your respiratory secretions drastically reduces. So the peak of someone’s infectivity is at the time of onset of showing symptoms and the viral loads or infectious virus particles in your respiratory fluid drastically reduces over time and can become 0 around day 9.
But even after 10 days from the onset of symptoms an RT-PCR can give positive results as this technology detect viral RNA irrespective of whether the nucleic acid is from a live virus or a dead virus. So, the PCR can give positive results for viral RNA in the nasal pharyngeal swab and in sputum for very long time. So, as you know in Sri Lanka until quite recent times, in order to discharge a patient from hospital you had to have two negative PCRs 24 hours apart. So, there have been instances where some people had to stay in the hospital for even 72 days without showing any symptoms. Just because of this scenario many psychological problems have been reported among those who had to be in hospital for longer periods.

( Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. 2020. [PMID:32374370] )
Researchers as early as in April and May this year have showed that the live virus was not identified in in respiratory secretions after 9 days from the onset of symptoms. So many studies appeared later proving the same. Sri Lanka took a decision to change discharge criteria for COVID patients based on these research findings and also observing other countries following such discharge criteria for many months. According to the new patient discharge guidelines asymptomatic or mildly symptomatic individuals are sent home after 14 days without doing a PCR. One of the research papers on this appeared on Nature Medicine in April, and it describe this scenario quite well.
According to the study a significant proportion of individuals, which is around 44 percent,begin spreading the virus before any symptoms appear. This study clearly shows that the viral load peaks 48 hours before the onset of symptoms and becomes almost zero after 8 to 10 days.

In another very intricate study done in Taiwan they show the family members, non-family members and health care personnel who came into contact with infected individuals who show symptoms within first two days have become infected with the virus. But those who came in to contact with the patient after 5 days from the onset of symptoms have not got the infection. So, these research clearly shows that it was during the first five days that these patients can mostly spread the infection. But this is only about patients with a simple or mild symptom and all those who are asymptomatic. Those who have pneumonia or those who have a weak immune system can carry and spread infectious viruses for weeks.
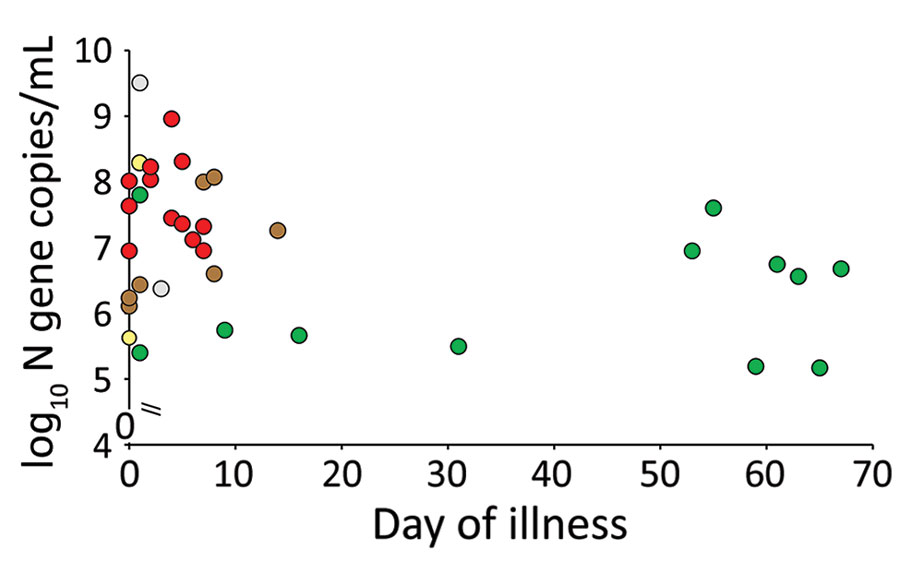
(Table – Perera, R., Tso, E., Tsang, O., Tsang, D., Fung, K., Leung, Y….Peiris, M. (2020). SARS-CoV-2 Virus Culture and Subgenomic RNA for Respiratory Specimens from Patients with Mild Coronavirus Disease. Emerging Infectious Diseases, 26(11), 2701-2704. https://dx.doi.org/10.3201/eid2611.203219.)
In another study published in Emerging Infectious Diseases from one of our own Sri Lankans Prof. Malik Peiris and his team have shown that the culturalable virus was basically found in patients sputum only within the seven days from the onset of symptoms. In this study they studied some patients with very high viral copy numbers even after 50, 60, or sometimes close to 70 days. But the most interesting thing they uncovered in this study is that even though these patients had very high viral copy numbers later on in illness, there were no infectious virus particles found in their sputum after 10 days. This was actually not a unique observation for SARS CoV 2 virus, viruses like SARS and MERS from the same viral family also have shown similar patterns. In this study it was also revealed that after 10 days infected people do shed the virus intermittently even though they are not infectious.
This also leads to a little bit of confusion in diagnostics. Because of this pattern although you have no infectious virus after 10 days PCR test gives a positive result and the PCR become negative and again gives positive reading after two three days. This positive but then negative pattern can go on for days or weeks and is called intermittent viral shedding, which was also seen with the SARS and MERS viruses, which are of the same family. There have been instances where we had to do 12 to 15 PCRs on the same person in order to discharge them as a COVID free individual. This phenomenon is well described in so many studies everywhere in the world.
Who Spreads the Virus?
With all the hype going around, many in the Sri Lankan society see COVID patients as terrorists. If you see them, you run a mile. But this study by Prof. Malik and his team which was published in Emerging Infectious Diseases clearly showed that 69 percent of patients with COVID 19 did not transmit the infection to anybody else. So just because you’re infected with the SARS CoV2, that doesn’t mean that you have scary amounts of virus in your respiratory secretions even if you are in the first five days or seven days of spreading the infection, you might not be spreading infection. But this does not mean you should not be isolated. If you have COVID19 it is very important to make sure you are isolated and take precautions not to spread the infection to others
As mentioned before about 69% of COVID cases did not transmit the infection to anybody but 19 percent of cases were responsible for 80% of the transmission. These 19% of COVID 19 careers who transmitted to 80% of people are called super spreaders.
Super Spreaders
Super spreader of COVID 19 can be defined as someone who has a very high infectious viral load in his/her respiratory secretions and produce smaller droplets of those secretions that travels relatively long way while they speak or cough.
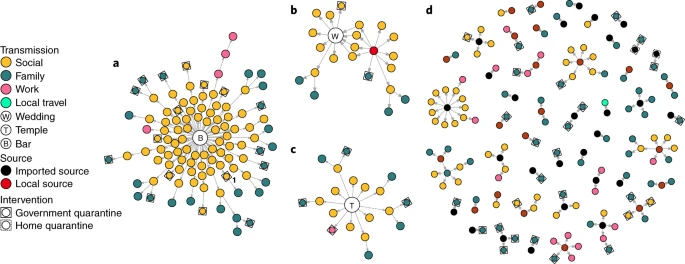
The above image from a recent study shows how different locations and different events fuel the COVID 19 transmission through the society. Studies like this have shown that closed indoor environments facilitate COVID transmission more than an outdoor environment. With the results of these studies we now know that being safe, is not just about keeping one meter or two visual distance. What is more important is where you actually go and where you are getting contacted more people. It’s evident that closed and crowded environments are ideal places for super spread event to happen and just need one super spreader to infect everybody in the room.
Sequencing, Why it’s important
Sequencing a viral genome is important in many ways. First, sequencing gives us the opportunity to understand the origin of a virus, so talking about SARS CoV 2 we now know that it came from a bat thanks to the complete genome sequencing of the virus.
And also, the sequencing helps us to understand how rapidly the virus mutate, Or so in the initial stages and even now you need to know how rapidly the virus is mutating and whether it has implications for transmission or virulence.
At the same time, sequencing can be used to get an idea how well we are containing a virus in a region or within a country.
This SARS CoV2 is quite a stable virus because if it mutates at a real slow rate which is just two nucleotides per month. This rate of mutation can be used to identify the age of a particular viral strain. You also can find if there are multiple introductions, identifying local transmission versus imported cases in a community and intensity of Community transmission through viral genome sequencing. So, sequencing is important not just to understand disease but for epidemiological purposes as well.
SARS-CoV 2 sequencing in Sri Lanka
At the Department of Immunology and Molecular Medicine in the University of Sri Jayawardenapura, we started sequencing viral strains right from the beginning at March. When the pandemic was unfolding here in Sri Lanka we saw different clusters and we acquired viral samples from each of those clusters and sequenced the genome.
We have also sequenced viral samples from currently ongoing outbreak as well. Our work is still ongoing and we keep sequencing different viral samples from different places of the outbreak to see what is going on and to see how the virus is evolving.
We upload our sequencing data to a website called nextstrain.org where they get sequencing data from different countries in the world. Through this website anyone can compare your viral sequences with the viral sequences all over the world making it easier to map the mutations happening in different places on the earth.

At the same time there is another website called GISAID, which is a similar website that act as a database for viral sequencing data. According to the GISAID there are seven major SARS nCoV 2 viral strains in the world. Out of those 7 in Sri Lanka we have identified four different strains of the COVID 19 causing virus (GH, GR, O and G).


The graphs above show how these GISAID strain distribution changed over time.
Classifying Viral Genome Mutations
Different institutes and websites in the world use different classifications for the mutations of the SARS nCoV 2 viral genome. As given above GISAID method is one such method in which all the mutations were grouped in to 7 different groups named GH, GR, G, V, S, O and L.
In Nextstrain.org the classify the mutations in to 5 different groups called clades named 19A, 19B, 20A, 20B and 20C. This website list all the mutations in a single phylogenic tree showing all the mutations in a timeline.
But one of the most detailed mutation classification method is the pangolin method. Pangolin is a phylogenic assignment of named Global outbreak lineages. So you can actually upload your sequences to this Pangolin web app, analyze and compare your sequencing data with present lineages. According to Pangolin classification there are two main Pangolin Lineages of SARS nCoV 2 virus named Lineage A and Lineage B, and then under each of these there are sub lineages named as A.1, A.2, A.3, and so on(Image).

In this website, they have lineage descriptions with from where everything comes from (Image). According to this Pangolin lineage classification the viruses from the current outbreak belongs to the B.1 lineage which is a large European lineage. But that doesn’t mean that this virus came from Europe at all. It just means that it was first described there and the virus of course may have come from somewhere else. So, these phylogenic classifications can’t be used to determine from where a virus came from to initiate an outbreak.
Main Mutations of SARS nCoV2

(Image – Nathan D. Grubaugh, William P. Hanage, Angela L. Rasmussen
Making Sense of Mutation: What D614G Means for the COVID-19 Pandemic
Remains Unclear
Cell, Volume 182, Issue 4, 20 August 2020, Pages 794-795)
In SAR-CoV 2 viral genome sequencing scientists have identified several main mutations that has happened over the time. In Sri Lankan viral strains we could identify two main mutations out of those named D614G and P1263L. From these two D614G mutation was the most common mutation that has been found all over the world. But later G614 variant immerged and gradually replaced the D614 variant. In Sri Lanka 24 viral samples out of 35 we have sequenced so far had this G614 mutation. And from the current outbreak, 15 samples out of 16 had the new mutation. The research work on this novel mutation have revealed that it is associated with lower RT-PCR cycle thresholds, suggestive of higher upper respiratory tract viral loads (Higher transmission ability), but not with increased disease severity.