Article by –

Introduction
Heavy metal pollution has become an ongoing environmental issue in the world. Increasing population growth and industrialization have resulted many organic & agricultural wastes and decaying urban infrastructure as soil and water pollution sources, especially in South Asian countries like India and Sri Lanka. Heavy metals such as Cu, Zn, Fe, Cd, Pb, Cr, Hg, and Sb are utilized as chemical additives for catalysis and coloring pigments in many industries including rubber, plastic, and paint. Cationic forms of these heavy metals can either be entered into human body through direct food chains or polluted water by leaching from agricultural soils. Exposure to elevated levels of ionic species of heavy metals such as As, Cd, Pd, Hg, Cr, Ni, Mn, and Fe in drinking water cause alterations in functional and structural integrity of kidney, liver, and brain. Inhabitants living especially in intense agricultural areas in North, North Central, North West, Eastern, Uva, and Central provinces in Sri Lanka suffer from health issues associated with consumption of heavy metal contaminated drinking water.
Recent research provides remediation methods for purifying polluted water bodies. These include filtration methods, reverse osmosis (RO), distillation, and chemical purification etc. These systems, mostly optimized for large-scale treatment plants, carry difficulties for operation, management, maintenance, and beyond the reach of consumer utilization. The work discussed here introduces low-cost, biodegradable, and 3D printed hydrogel material that captures toxic heavy metal ions from contaminated water. Cost effectiveness and environmental friendliness of the material would be beneficial for Sri Lankan consumers to be used in their house-hold water filtration systems.
Biopolymer-based 3D Printed Hydrogel Cups
Unlike injection molding, 3D printing allows fabrication of complex geometries with increased surface areas. In this work, direct ink-writing (DIW) 3D printing technique is used for fabricating the 3D cup-shape structures (Figure 1). A biopolymer (BP) containing chelation sites for heavy metal ions (i.e. Pb2+, Cu2+, Cd2+, and Hg2+) is mixed with a photo-crosslinkable polymer (PCP) in ultrapure water (UPW). This mixture, formed into a shear-thinning hydrogel at room temperature, is loaded in to the DIW syringe and pressurized to form the 3D structures layer-by-layer fashion. The hydrogel formed by mixing PCP and UPW is used as the control. All structures are cured via UV immediately after printing to provide additional strength.
Heavy Metal Ion Adsorption Tests The 3D printed cups are filled with aqueous solutions of each metal ion in concentrations that represent moderately polluted water bodies. For the detection of Cu2+ levels, a 1500 ppb Cu2+ stock solution (5 mL) is prepared using the nitrate salt in UPW and added to the cups, each of which contains 3 g of dry hydrogel, followed by shaking at pH7.0 and room temperature for 30, 60, 90 and 120 min. After each time period, the supernatant is analyzed via inductively coupled plasma-mass spectrometry (ICP-MS). To detect Cd2+ and Pb2+ levels, the same procedure is carried out but using 30 ppb stock solutions, Hg2+ levels with a 10 ppb stock solution, prepared using the corresponding metal nitrate salt. The BP/PCP/UPW cups show significantly larger adsorption compared to PCP/UPW, the control, reducing the concentration of each metal below the environmental protection agency (EPA) action level, after only 30 min of contact time. Since EPA action level is defined as the maximum pollutant concentration that is considered safe, the fabricated cups could produce safe drinking water (Figure 1) within 30 min of contact with the pollutants.
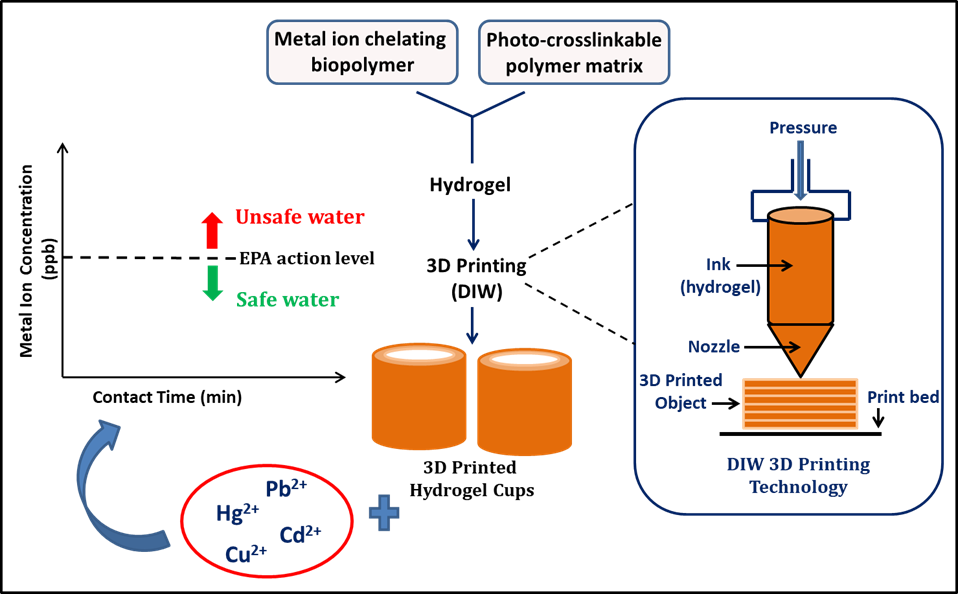
Figure 1. Preparation of the hydrogel, direct-ink write (DIW) 3D printing process of the hydrogel cups, and heavy metal ion adsorption tests set-up with the printed cups. Adapted from Ref (1).
Efficient Removal of Pb2+
Pb2+ can leach from plumbing systems of public water supplies and is the most common metal pollutant in drinking water of the four metals tested here. In order to evaluate the effects of surface area on the adsorption kinetics, we carry out tests using aqueous solutions of Pb2+ ions using shapes consisting of BP/PCP/UPW with different amounts of accessible surface areas (Figure 2A). These shapes (2 g dry hydrogel) are submerged in 30 ppb lead nitrate stock solutions (5 mL) in UPW and shaken for 10 min at room temperature. The remaining Pb2+ in each solution is measured with ICP-MS. According to the results, Pb2+ removal efficiency has been improved with increasing surface area (Figure 2B). The BP/PCP/UPW-based shape B is capable of removing ca 74% of the Pb2+ ions after 10 min, ca four times more than that of its PCP/UPW counterpart.
Reusability Tests Finally, reusability tests are also performed for the 3D printed BP/PCP/UPW-based shape B (Figure 2C). The used adsorbent is first washed with an aqueous HCl (1mol L–1) solution (5mL) until no more Pb ions are detected, followed by neutralization with an aqueous NaOH (0.1 mol L–1) solution (5 mL) several times and UPW until the pH of the filtrate is 7. The adsorbent is then subjected to Pb2+ adsorption again by soaking in 30 ppb stock solution (5 mL) in UPW and placed on a shaker at room temperature for 10 min. The remaining Pb2+ level is measured using ICP-MS. This procedure is repeated five times. We observe that ca 98% recovery of the original Pb2+ removal efficiency can be achieved even after five cycles with an overall structural integrity of the adsorbent.
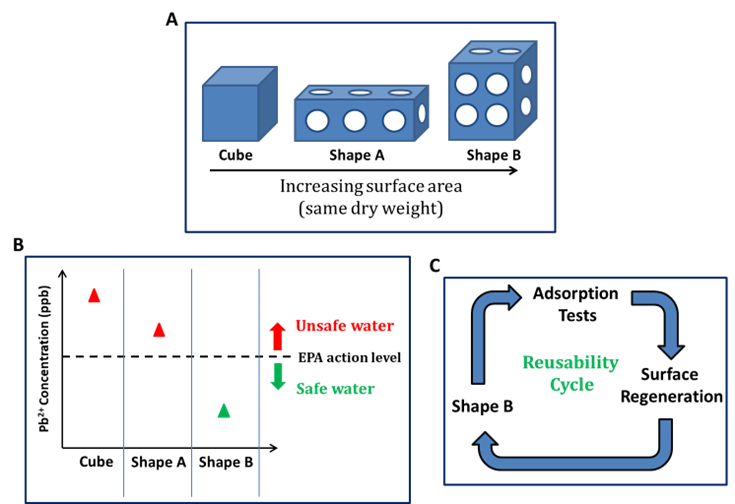
Figure 2. 3D printed hydrogel structures with increasing surface area (left to right) (A). Pb2+ adsorption testing with printed structures (B). Reusability cycle set-up with printed shape B (C). Adapted from Ref (1).
Conclusion
In conclusion, we have designed recyclable, 3D printable materials from a cheap, abundant BP for the remediation of toxic heavy metal ions in water. Using these BP/PCP/UPW materials, we are able to successfully prepare 3D printed objects including cups and objects with more complex internal structures, which are difficult to obtain via conventional moulding techniques. Moreover, this novel and cost-effective approach to remove health and environmental hazards could be useful for fabricating cheap and safe water filtration devices on site in polluted areas in countries like Sri Lanka without the need for industrial scale manufacturing tools. Further research is being carried out on developing materials processable via more affordable 3D printing technologies. Moreover, novel policy decisions that encourage Sri Lankan community towards such adsorbents would be highly beneficial to overcome health issues associated with metal polluted water.
Reference:
(1) Appuhamillage GA, Berry DR, Benjamin CE, Luzuriaga MA, Reagan JC, Gassensmith JJ, et al. A biopolymer-based 3D printable hydrogel for toxic metal adsorption from water. Polym Int. 2019;68: 964–71.




